When dealing with flammable substances, understanding their ignition characteristics is critical for ensuring safety in handling, storage, and transportation. Three fundamental thermal properties that define the fire hazard of flammable liquids are Flash Point, Fire Point, and Auto Ignition Temperature. These properties help assess how easily a substance can catch fire and how it behaves in the presence (or absence) of an ignition source.
Contents
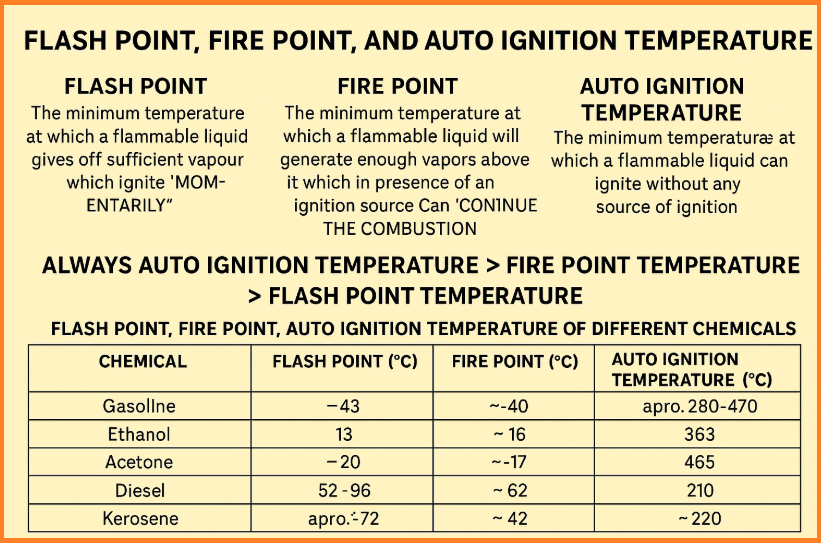
1. Flash Point
Definition:
The Flash Point is the minimum temperature at which a flammable liquid emits enough vapor to form a combustible mixture with air that ignites momentarily when exposed to an ignition source (such as a flame or spark). However, the combustion is not sustained.
Key Characteristics:
- Indicates volatility and fire hazard.
- Lower flash points indicate higher flammability.
- Measured using standard apparatus such as the Pensky-Martens or Abel closed cup testers.
Example Flash Points:
| Chemical | Flash Point (°C) |
|---|---|
| Gasoline | -43 |
| Ethanol | 13 |
| Acetone | -20 |
| Diesel | 52–96 |
| Kerosene | ~38–72 |
2. Fire Point
Definition:
The Fire Point is the minimum temperature at which a flammable liquid emits vapors that, upon ignition, continue to burn for at least five seconds. It is usually a few degrees higher than the flash point and reflects the ability of the liquid to sustain combustion.
Key Characteristics:
- Determines the actual fire risk of a substance under sustained conditions.
- Useful in fire safety and storage guidelines.
Example Fire Points:
| Chemical | Fire Point (°C) |
|---|---|
| Gasoline | -40 (approx.) |
| Ethanol | ~16 |
| Acetone | ~-17 |
| Diesel | ~62 |
| Kerosene | ~42 |
3. Auto Ignition Temperature (AIT)
Definition:
The Auto Ignition Temperature is the minimum temperature at which a flammable liquid or vapor will spontaneously ignite without any external ignition source. This temperature is critical for understanding the risks of spontaneous combustion, especially in industrial settings.
Key Characteristics:
- Much higher than flash and fire points.
- Independent of external flames or sparks.
- Important for process safety and hazard analysis.
Example Auto Ignition Temperatures:
| Chemical | Auto Ignition Temperature (°C) |
|---|---|
| Gasoline | ~280–470 |
| Ethanol | 363 |
| Acetone | 465 |
| Diesel | 210 |
| Kerosene | ~220 |
Comparative Relationship
The typical order of these temperatures for any flammable liquid is:
Auto Ignition Temperature > Fire Point > Flash Point
This reflects the increasing level of thermal energy needed for:
- Vapor to momentarily ignite (Flash Point),
- Vapor to sustain combustion (Fire Point), and
- Vapor to ignite spontaneously (Auto Ignition Temperature).